Macrophage Growth Factor Ligands/Receptors: Regulators of Autoimmune Disease
Nephritis is a major cause of morbidity and mortality in lupus and occurs in a large proportion of patients with lupus. Even with optimal therapy, many patients progress to end stage renal disease. As a new FDA approved therapeutic for lupus nephritis has not been approved in over five decades, the need for a novel therapeutic target is pressing and timely.
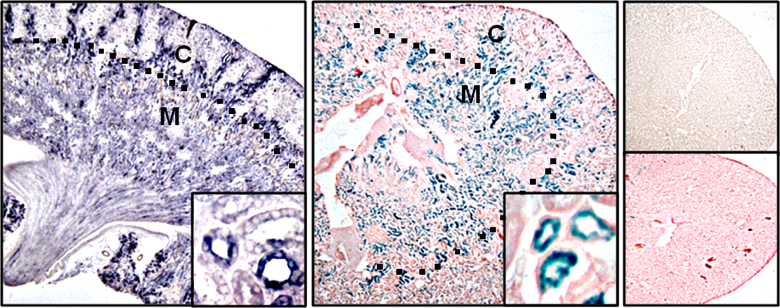
Myeloid cells, most notably macrophage regulate the inflammatory response to injury. Macrophages are key orchestrators of inflammation mediating tissue repair and conversely, non-resolving inflammation leading to chronic disease. Activated macrophages are broadly divided into “destroyers” and “healers”. Owing to extreme plasticity and exquisite sensitivity to the microenvironment, activated macrophage phenotypes do not crisply fall into this simple framework, but rather lie within a continuum along a spectrum, with destroyer and healers phenotypes at opposite ends. It is well established that macrophages are integral in acute kidney injury that resolves in normal mice, but triggers chronic kidney disease in lupus-prone mice. Therefore, the principal molecules required for macrophage survival and proliferation, colony stimulating factor 1 (CSF-1) and the recently identified IL-34, are central to regulating the fate of the inflamed kidney. Our projects explore the mechanisms central to IL-34 and CSF-1 and their cognate receptors in the pathogenesis of lupus nephritis. Within this framework we are probing for the triggers evoking chronic kidney diseases.
Rheumatoid arthritis (RA) is common chronic inflammatory, destructive joint disease of uncertain etiology that creates a prodigious financial burden for patients and society. While current biologic agents revolutionized the treatment of RA, far too many patients are poorly or non-responsive to these agents and current therapeutic approaches often cause off-target adverse effects. Thus, there is a pressing need to pinpoint the pathogenic mechanisms central to RA, identify predictive biomarkers to guide individualized, accurate treatment and novel therapeutic targets. We are funded for projects addressing each of these aspects within the context of myeloid mediated pathways.
Limb-girdle muscular dystrophy type 2B or Miyoshi muscular dystrophy 1 (LGMD2B-Miyoshi) is an autosomal recessive neuromuscular disorder caused by a deficiency of functional dysferlin protein. This illness is characterized by progressive skeletal muscle wasting that often begins suddenly in early adulthood and renders the patient immobile. As there currently is no effective treatment for this disease, deciphering the pathogenesis of this illness is central to uncovering novel therapeutic targets.
Macrophages are prominent in skeletal muscles in patients with LGMD2B-Miyoshi. We are testing the hypothesis that an unchecked expansion of novel destructive macrophages drives unrelenting muscle injury that advances this form of muscular dystrophy. As we are pinpointing the macrophage-mediated mechanisms instrumental in the pathogenesis of this illness, we are likely to uncover novel therapeutic strategies.
